Meditation experience is associated with increased cortical thickness
Meditation experience is associated with increased cortical thickness
Sara W. Lazar,[1] Catherine E. Kerr,[2] Rachel H. Wasserman,[1] [2] Jeremy R. Gray,[3] Douglas N. Greve,[4] Michael T. Treadway,[1] Metta McGarvey,[5] Brian T. Quinn,[4] Jeffery A. Dusek,[6][7] Herbert Benson,[6][7] Scott L. Rauch,[1] Christopher I. Moore,[8][9] and Bruce Fischl[4][10]
Abstract
Previous research indicates that long-term meditation practice is associated with altered resting electroencephalogram patterns, suggestive of long lasting changes in brain activity. We hypothesized that meditation practice might also be associated with changes in the brain’s physical structure. Magnetic resonance imaging was used to assess cortical thickness in 20 participants with extensive Insight meditation experience, which involves focused attention to internal experiences.
Brain regions associated with attention, interoception and sensory processing were thicker in meditation participants than matched controls, including the prefrontal cortex and right anterior insula. Between-group differences in prefrontal cortical thickness were most pronounced in older participants, suggesting that meditation might offset age-related cortical thinning. Finally, the thickness of two regions correlated with meditation experience. These data provide the first structural evidence for experience-dependent cortical plasticity associated with meditation practice.
Keywords: insula, meditation, plasticity, prefrontal cortex
Introduction
Meditation is a form of mental exercise that has become a popular US health practice. Regular practice of meditation is reported to produce changes in mental state and resting electroencephalogram patterns that persist beyond the time-period of active practice [11]. We hypothesized that regular meditation practice should also result in significant changes in the cortical structure in regions that are routinely engaged during this mental exercise. To test this hypothesis, we used magnetic resonance imaging to visualize differences in the thickness of the cerebral cortex of experienced Buddhist Insight meditation practitioners.
This form of meditation does not utilize mantra or chanting. Rather, the main focus of Insight meditation is the cultivation of attention and a mental capacity termed ‘mindfulness’, which is a specific nonjudgmental awareness of present-moment stimuli without cognitive elaboration [12].
Formal practice involves sustained mindful attention to internal and external sensory stimuli. Thus, we tested the hypothesis that between-group and experience-dependent differences in cortical thickness would be found in brain regions involved in attention and sensory processing, thereby showing evidence of cortical plasticity.
Participants and methods
Twenty participants with extensive training in Insight meditation were recruited from local meditation communities. These participants were not monks, but rather typical Western meditation practitioners who incorporate their practice into a daily routine involving career, family, friends and outside interests.
Two participants were full-time meditation teachers, three were part-time yoga or meditation teachers and the rest meditated an average of once a day for 40 min, while pursuing traditional careers in fields such as healthcare and law. On average, participants had 9.1 ± 7.1 years of meditation experience and practiced 6.2 ± 4.0 h per week. Participants were required to have participated in at least 1 week-long Insight meditation retreat, which entails approximately 10 h of meditation per day. Fifteen control participants with no meditation or yoga experience were also recruited.
The meditation and control participants were matched for sex (meditators 65% male, controls 67%), age (meditators 38.2 years old, controls 36.8 years old), race (both groups 100% Caucasian) and years of education (meditators 17.3 years, controls 17.4 years). All participants were physically and psychologically healthy.
Two meditation participants were left-handed; exclusion of the left-handed participants did not significantly alter results. All participants provided written, informed consent and the study was approved by the Institutional Review Board at the Massachusetts General Hospital.
The present methods utilized a well-validated computational approach to measure the thickness of the cerebral cortex [13][14].
Cortical thickness was estimated from two magnetization prepared rapid gradient echo (MPRAGE) structural images collected from each participant that were then motion-corrected and averaged together to form a single high-resolution image [13][15]. An initial estimate of the gray/white matter boundary was constructed by classifying all white matter voxels in a magnetic resonance imaging volume using a combination of geometric and intensity-based information.
A surface-deformation procedure was then used to obtain subvoxel resolution in the gray/white boundary and in the pial surface using a combination of smoothness constraints and intensity terms. The resulting cortical surface models for all participants were aligned to an atlas of cortical folding patterns using a high-dimensional nonlinear registration technique.
Results
The mean thickness across the entire cortex did not differ significantly between the groups for either hemisphere (P>0.10), indicating that it was not the case that the cortex of meditators is nonspecifically thicker everywhere. Statistical thickness-difference maps constructed using the Kolmogorov-Smirnoff statistics (one-tailed, α-level P=0.05), however, indicated that significant differences in the ‘distribution’ of thickness existed between groups across both hemispheres (k=3.89, P=0.0001), and in each hemisphere separately (k=3.02, P=0.0025 for left hemisphere; k=2.49, P=0.013 for right hemisphere).
This finding indicates that the pattern of relative thickness across each hemisphere was different between groups. Protected by significant unidirectional results for the omnibus test for each hemisphere, an unpaired t-test was performed to test for specific loci of significant between-group differences in regional cortical thickness. Specifically, we tested the a priori hypotheses that differences would be observed within prefrontal, interoceptive and unimodal sensory cortical regions.
A false discovery rate of 0.05 corresponding to an uncorrected P=3.5 × 10-4 was used to correct for multiple comparisons [16].
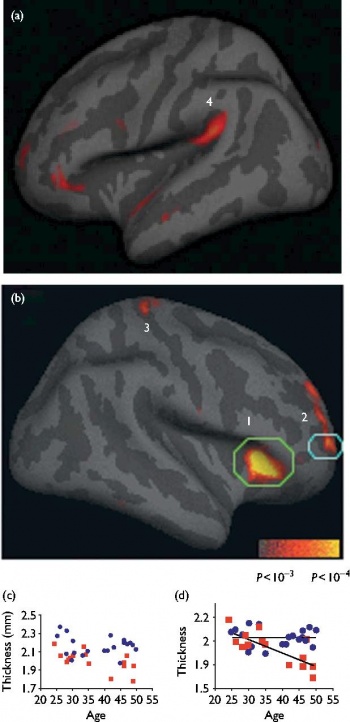
Within the search territory, a large region of right anterior insula (P=1.2 × 10-5) and right middle and superior frontal sulci corresponding approximately to Brodmann areas (BA) 9 and 10 (P=1.8 × 10-5) were significantly thicker in meditators than in controls (Fig. 1). The left superior temporal gyrus (auditory cortex, P=3.7 × 10-4) and a small region in the fundus of the central sulcus, (BA 3a, somatosensory cortex, P=6.0 × 10-4) showed trends towards a significantly thicker cortex in meditation participants than in controls.
Analysis of the right frontal BA 9/10 subregion resulted in a significant age by group interaction, F(1,31)=10.85, P=0.002, with typical age-related decreases observed in the control group [r(13)=-0.76, P=0.001] but not in the meditation group [r(18)=-0.05, P=0.83]. Significant interactions were not observed in any other brain region.
As a further confirmation that meditation can influence experience-dependent plasticity, we tested whether objective measures of meditation experience correlated with cortical thickness. As frequency of daily practice varies between meditation practitioners, using the total number of years of practice is not a sensitive metric of experience. One effect of regular meditation practice is a significant drop in respiration rate during formal practice [17][18]. We therefore tested whether changes in respiration rate between rest and meditation could serve as an objective measure of meditation experience.
The change in mean respiration rate from a 6-min baseline period to the first 6 min of the meditation period was calculated for each participant and then correlated with the self-reported total number of hours of formal sitting meditation over the participant’s lifetime (r=-0.75, P<0.001). The correlation between respiration rate and total number of years the participant had been practicing was also significant (r=-0.57, P=0.009); however, the correlation with total hours of formal sitting practice resulted in a higher coefficient.
To directly test for cumulative effects of meditation experience on brain structure, a correlation was performed between cortical thickness and change in respiration rate. After correcting for multiple comparisons using a false discovery rate associated with a P=0.05, the analysis revealed one significant region within the inferior occipito-temporal visual cortex (Fig. 2).
Among the meditation group, the zero-order correlation between thickness in this region and change in respiration rate was r(18)=0.72, P<0.001, which was effectively unchanged when controlling for individual right-hemisphere mean thickness (as a measure of nonspecific effects on cortical thickness), partial r(17)=0.73, P<0.001, and still further when controlling for age, partial r(16)=0.75, P<0.001.
When controlling for age and individual right-hemisphere average thickness, a partial correlation between thickness in this region and years of experience remained significant [partial r(15)=0.627, P=0.007]. These findings are consistent with the hypothesis that meditation practice promoted thickening in this region.

Visual area correlated with meditation experience. (a) Statistical map depicting cortical thickness correlated with change in respiration rate. (b) Scatter plot of mean cortical thickness of each participant from the circled region within the inferior ...
The most experienced participants were also among the oldest. As age-related decreases in cortical thickness are greatest in frontal regions [15], it is possible that the effect of age may obscure the modest effects of meditation practice in these areas. The Pearson correlations between respiration rate and cortical thickness in the insula and BA 9/10 were not significant (r=-0.36, P=0.12 and r=-0.23, P=0.33, respectively), although they became so in the insula after controlling for age [partial r(17)=0.48, P=0.04]. The correlation between these parameters for the BA 9/10 region was essentially unchanged [partial r(17)=-0.25, P=0.30].
Discussion
Our data indicate that regular practice of meditation is associated with increased thickness in a subset of cortical regions related to somatosensory, auditory, visual and interoceptive processing. Further, regular meditation practice may slow age-related thinning of the frontal cortex.
Previous studies of cortical plasticity in animals and humans have shown that when a task requires that attention be consistently directed towards a behaviorally relevant sensory stimulus (e.g. a somatosensory [19] or auditory stimulus [20]) over repeated practice sessions [21], robust changes in sensory cortical maps result ([22] and Kerr CE, Wasserman RH and Moore CI. Cortical plasticity as a therapeutic mechanism for touch healing, under reveiw).
Additional studies suggest that relaxation facilitates the learning-based process that underlies such cortical plasticity [23].
It may be useful to conceptualize meditation practice as engaging in an analogous set of cortical remodeling processes: namely, directing attention towards behaviorally relevant sensory stimuli within a relaxing setting over repeated practice sessions [12][17].
Increased cortical thickness could be due to greater arborization per neuron, increased glial volume or increased regional vasculature. The methods employed do not distinguish between these possibilities; however, each of these mechanisms is supportive of increased neural function.
We hypothesized that meditation practice should promote neural plasticity in regions that are routinely engaged during formal practice. Many factors including age, sex, genetics, neuropathology and psychopathology [14][15][24][25], however, influence the thickness of cortex nonspecifically, confounding these analyses.
Perhaps the largest of these confounds is the effect of age. The rate of age-dependent thinning is highly variable across the cortical surface [15]. Meditation-related effects on thickness may have been counterbalanced by the effects of age on cortical thinning, thereby minimizing our ability to detect significant correlations.
Thinning is most pronounced in the frontal lobe, and indeed there were many regions in the parietal, temporal and occipital lobe where there was little if any difference in the average thickness in our older and younger participants (data not shown). Such age-related effects may account for the fact that the strongest correlation with experience was found in the occipitotemporal region, while other regions of interest, which all lie in frontal regions, had only low correlation with experience.
Interestingly, despite the effects of aging on the prefrontal cortex, in one focal region of BA 9/10 the average cortical thickness of the 40-50-year-old meditation participants was similar to the average thickness of the 20-30-year-old meditators and controls, suggesting that regular practice of meditation may slow the rate of neural degeneration at this specific locus. Future longitudinal studies will be required to verify this finding.
Another factor possibly confounding our ability to detect correlations between thickness and experience is heterogeneity in the specific mental exercises that Insight practitioners engage in over time. Beginners are taught to maintain focused awareness on interoceptive stimuli and then are gradually taught to expand their awareness to focus on thoughts, emotions and external stimuli such as sounds, although there is no prescribed schedule or order in which these practices are taught.
Correspondingly, the insula, an area associated with the interoceptive processes and breath awareness techniques common to beginning and experienced meditators, had the largest and most significant between-group difference, while unimodal sensory areas, which may be associated with more advanced and heterogeneous practices, had less significant differences.
As a result of the cross-sectional nature of the study, the findings are necessarily correlational, and a causal relationship between cortical thickness and meditation cannot be inferred. For example, it is possible that people with thicker sensory cortex are for some reason drawn to meditation. Several factors, however, suggest that these findings relate to the meditative practice itself.
First, although there were significant ‘regional’ differences in thickness between groups, there was no between-group difference in ‘global’ mean cortical thickness, indicating that these findings are unlikely to be due to spurious between-group differences that might impact cortical structure nonspecifically.
Second, the regions of cortical thickening correspond well to the specific activities that practitioners of Insight repeatedly engage in over time - paying attention to breathing sensations and sensory stimuli. It is unlikely that nonspecific lifestyle effects such as diet would be associated with the specific pattern of differences found. The most plausible explanation for the specific pattern observed is experience-dependent cortical plasticity.
Finally, both years of practice and change in respiration rate (a physiological measure of cumulative meditation experience) were correlated with cortical thickness in two regions, the inferior occipitotemporal visual cortex and right anterior insula.
These findings are consistent with other cross-sectional reports of experience-dependent differences in neural volume [26] [27]. In addition, a longitudinal study [28] has demonstrated that learning to juggle is associated with increases in visual motion cortical areas. Our finding of a correlation between the thickness in two regions and amount of experience lends support to the hypothesis that the observed differences are acquired through extensive practice of meditation, and are not simply due to preexisting or incidental between-group differences.
Most of the regions identified in this study were found in the right hemisphere. The right hemisphere is essential for sustaining attention [29], which is a central practice of Insight meditation. The largest between-group difference was in the thickness of right anterior insula.
Functional imaging and electrophysiological studies in humans and monkeys have implicated the right anterior insula in tasks related to bodily attention and increased visceral awareness [30][31]. Structural measures of gray matter volume of the right anterior insula predict accuracy of objective measures of interoceptive performance, as well as subjective ratings of global visceral awareness [31].
The differential thickness between groups in this region is consistent with increased capacity for awareness of internal states by meditators, particularly awareness of breathing sensations. Right BA 9/10 has been shown to be involved in the integration of emotion and cognition [32].
It has been hypothesized that by becoming increasingly more aware of sensory stimuli during formal practice, the meditation practitioner is gradually able to use this self-awareness to more successfully navigate through potentially stressful encounters that arise throughout the day [12][33].
This eastern philosophy of emotion dovetails with Damasio’s theory that connections between sensory cortices and emotion cortices play a crucial role in processing of emotionally salient material and adaptive decision making [34].
Other forms of yoga and meditation will likely have a similar impact on cortical structure, although each tradition would be expected to have a slightly different pattern of cortical thickening based on the specific mental exercises involved [17][18][35].
Although numerous studies have shown that indices of cortical size can decrease as a result of aging and pathology (e.g. [14][15]), there are limited data indicating mechanisms that promote cortical thickening [26][27][28]. Our findings suggest that cortical plasticity can occur, in adults, in areas important for cognitive and emotional processing.
Conclusion
Our initial results suggest that meditation may be associated with structural changes in areas of the brain that are important for sensory, cognitive and emotional processing. The data further suggest that meditation may impact agerelated declines in cortical structure.
Acknowledgements
We thank R. Gollub, D. Salat, M. Bar, G. Kuperberg and S. Stufflebeam for helpful discussions. We also thank I. Rosman for technical assistance, J. Zaki for manuscript editing, and D. Salat and D. Rosas for access to data.
Footnotes
Sponsorship:This work was supported by NIH/NCCAMK01AT00694-01, NCRR (P41RR14075), the MINDInstitute, and CDCGrants H75/CCH119124 and H75/CCH123424.C.K. was supported by Grant R21AT002860-02.
Footnotes
- ↑ 1.0 1.1 1.2 1.3 Psychiatric Neuroimaging Research Program, Massachusetts General Hospital
- ↑ 2.0 2.1 Osher Institute, Harvard Medical School, Boston, Massachusetts
- ↑ Department of Psychology, Yale University, New Haven, Connecticut
- ↑ 4.0 4.1 4.2 Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Boston
- ↑ Graduate School of Education, Harvard University, Cambridge
- ↑ 6.0 6.1 Mind/Body Medical Institute, Chestnut Hill
- ↑ 7.0 7.1 Department of Medicine, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston
- ↑ Department of Brain and Cognitive Sciences, Massachusetts Institute of Technology
- ↑ McGovern Institute for Brain Research
- ↑ Computer Science and AI Lab (CSAIL), Massachusetts Institute of Technology, Cambridge, Massachusetts, USA.
- ↑ Long-term meditators self-induce high-amplitude gamma synchrony during mental practice.
Lutz A, Greischar LL, Rawlings NB, Ricard M, Davidson RJ. Long-term meditators self-induce high-amplitude gamma synchrony during mental practice. Proc Natl Acad Sci USA. 2004;101:16369–16373. (PMC free article) (PubMed) - ↑ 12.0 12.1 12.2 Goldstein J, Kornfield J. Seeking the heart of wisdom: The path of Insight Meditation. Shambhala Publications; Boston: 1987.
- ↑ 13.0 13.1 Measuring the thickness of the human cerebral cortex from magnetic resonance images.
Fischl B, Dale AM
Proc Natl Acad Sci U S A. 2000 Sep 26; 97(20):11050-5.(PMC free article) (PubMed) - ↑ 14.0 14.1 14.2 Regional and progressive thinning of the cortical ribbon in Huntington's disease.
Rosas HD, Liu AK, Hersch S, Glessner M, Ferrante RJ, Salat DH, van der Kouwe A, Jenkins BG, Dale AM, Fischl B
Neurology. 2002 Mar 12; 58(5):695-701.(PubMed) - ↑ 15.0 15.1 15.2 15.3 15.4 Thinning of the cerebral cortex in aging.
Salat DH, Buckner RL, Snyder AZ, Greve DN, Desikan RS, Busa E, Morris JC, Dale AM, Fischl B
Cereb Cortex. 2004 Jul; 14(7):721-30. (PubMed) - ↑ Thresholding of statistical maps in functional neuroimaging using the false discovery rate.
Genovese CR, Lazar NA, Nichols T
Neuroimage. 2002 Apr; 15(4):870-8. (PubMed) - ↑ 17.0 17.1 17.2 Wallace RK, Benson H, Wilson AF. A wakeful hypometabolic physiological state. Am J Physiol. 1971;221:795–799. (PubMed)
- ↑ 18.0 18.1 Zazen and cardiac variability.
Lehrer P, Sasaki Y, Saito Y
Psychosom Med. 1999 Nov-Dec; 61(6):812-21. (PubMed) - ↑ Topographic reorganization of the hand representation in cortical area 3b owl monkeys trained in a frequency-discrimination task.
Recanzone GH, Merzenich MM, Jenkins WM, Grajski KA, Dinse HR
J Neurophysiol. 1992 May; 67(5):1031-56. (PubMed) - ↑ Temporal plasticity in the primary auditory cortex induced by operant perceptual learning.Bao S, Chang EF, Woods J, Merzenich MM
Nat Neurosci. 2004 Sep; 7(9):974-81. (PubMed) - ↑ Mapping perception to action in piano practice: a longitudinal DC-EEG study.Bangert M, Altenmüller EO
BMC Neurosci. 2003 Oct 15; 4():26. (PMC free article) (PubMed) - ↑ Merzenich MM, DeCharms RC. Neural representations, experience and change. MIT Press; Boston: 1996.
- ↑ Sleep and rest facilitate auditory learning.
Gottselig JM, Hofer-Tinguely G, Borbély AA, Regel SJ, Landolt HP, Rétey JV, Achermann P
Neuroscience. 2004; 127(3):557-61. (PubMed) - ↑ Regionally localized thinning of the cerebral cortex in schizophrenia.
Kuperberg GR, Broome MR, McGuire PK, David AS, Eddy M, Ozawa F, Goff D, West WC, Williams SC, van der Kouwe AJ, Salat DH, Dale AM, Fischl B
Arch Gen Psychiatry. 2003 Sep; 60(9):878-88. (PubMed) - ↑ A magnetic resonance imaging study of cortical thickness in animal phobia.
Rauch SL, Wright CI, Martis B, Busa E, McMullin KG, Shin LM, Dale AM, Fischl B
Biol Psychiatry. 2004 May 1; 55(9):946-52. (PubMed) - ↑ 26.0 26.1 Navigation-related structural change in the hippocampi of taxi drivers.
Maguire EA, Gadian DG, Johnsrude IS, Good CD, Ashburner J, Frackowiak RS, Frith CD
Proc Natl Acad Sci U S A. 2000 Apr 11; 97(8):4398-403. (PMC free article) (PubMed) - ↑ 27.0 27.1 Mechelli A, Crinion JT, Noppeney U, O’Doherty J, Ashburner J, Frackowiak RS, Price CJ. Neurolinguistics: structural plasticity in the bilingual brain. Proficiency in a second language and age at acquisition affect grey-matter density. Nature. 2004:431.
- ↑ 28.0 28.1 Neuroplasticity: changes in grey matter induced by training.
Draganski B, Gaser C, Busch V, Schuierer G, Bogdahn U, May A
Nature. 2004 Jan 22; 427(6972):311-2. (PubMed) - ↑ Review The attention system of the human brain.
Posner MI, Petersen SE
Annu Rev Neurosci. 1990; 13():25-42. (PubMed) - ↑ Review Interoception: the sense of the physiological condition of the body.
Craig AD
Curr Opin Neurobiol. 2003 Aug; 13(4):500-5. (PubMed) - ↑ 31.0 31.1 Neural systems supporting interoceptive awareness.
Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ
Nat Neurosci. 2004 Feb; 7(2):189-95. (PubMed) - ↑ Integration of emotion and cognition in the lateral prefrontal cortex.
Gray JR, Braver TS, Raichle ME
Proc Natl Acad Sci U S A. 2002 Mar 19; 99(6):4115-20. (PMC free article) (PubMed) - ↑ Segal ZV, Williams JMG, Teasdale JD. Mindfulness-based cognitive therapy for depression: a new approach to preventing relapse. Guilford Press; New York: 2002.
- ↑ Review The somatic marker hypothesis and the possible functions of the prefrontal cortex.
Damasio AR
Philos Trans R Soc Lond B Biol Sci. 1996 Oct 29; 351(1346):1413-20. (PubMed) - ↑ Functional brain mapping of the relaxation response and meditation.
Lazar SW, Bush G, Gollub RL, Fricchione GL, Khalsa G, Benson H
Neuroreport. 2000 May 15; 11(7):1581-5. (PubMed)